Hardness (Calcium) Titrets for EGTA Method
Best Price:
Buy Hardness (Calcium) Titrets for EGTA Method for $42.51 at @ Megadepot.com
No coupon is required — this is the standard retail price.
Set a price drop alert to never miss an offer.
Price Comparison
| Seller | Contact Seller | List Price | On Sale | Shipping | Best Promo | Final Price | Volume Discount | Financing | Availability | Seller's Page |
|---|---|---|---|---|---|---|---|---|---|---|
|
BEST PRICE 1 Product Purchase
|
   |
$45.08 | $42.51 |
|
$42.51 | See Site | Visit Store |
Product Details
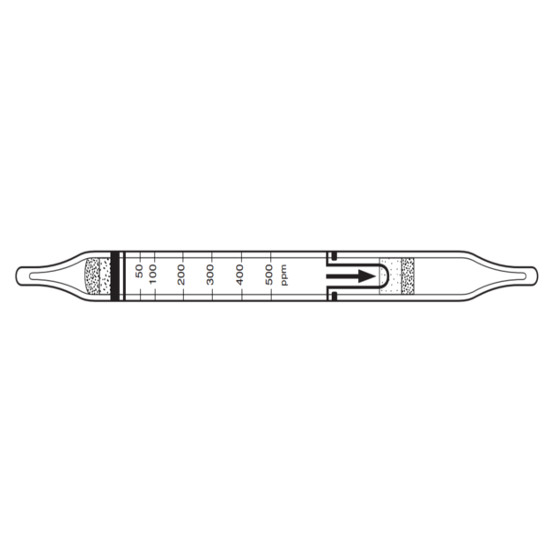
Chemetrics, K-1705, 50-500ppm Hardness (Calcium) Titrets for EGTA Method, KitFeatures:Method: 50 ppm.Range: 50-500 ppm as CaCO3.Increments: 50, 55, 60, 65, 70, 75, 80, 90, 100, 125, 150, 175, 200, 250, 350, 500 ppm.Shelf-life 8 months.Kit comes in a cardboard box and contains everything needed to perform 30 tests: thirty ampoules with valve assemblies, Indicator Solution, titrettor, 25 mL sample cup and instructions.MethodHardness is a measure of the mineral content of water. Calcium and magnesium are the most common minerals that contribute to hardness. Hard water causes scaling in boilers and other industrial equipment, and diminishes the effectiveness of soaps and detergents.The EGTA Method (calcium)The EGTA method is specific for calcium hardness. The EGTA titrant in alkaline solution is employed with a zincon indicator. Results are expressed as ppm (mg/L) CaCO3. Shelf-life: eight months. Although the reagent itself is stable, the end point indicator has a limited shelflife. We recommend stocking quantities that will be used within seven months.The EDTA Method (total)The total hardness method is applicable to drinking, surface, boiler, and brine waters. The EDTA titrant is employed in alkaline solution with a calmagite indicator. This method determines the combined calcium and magnesium concentration of a sample. If no magnesium is present, the end point of the titration normally appears sluggish. Results are expressed as ppm (mg/L) CaCO3.

 Copied
Copied